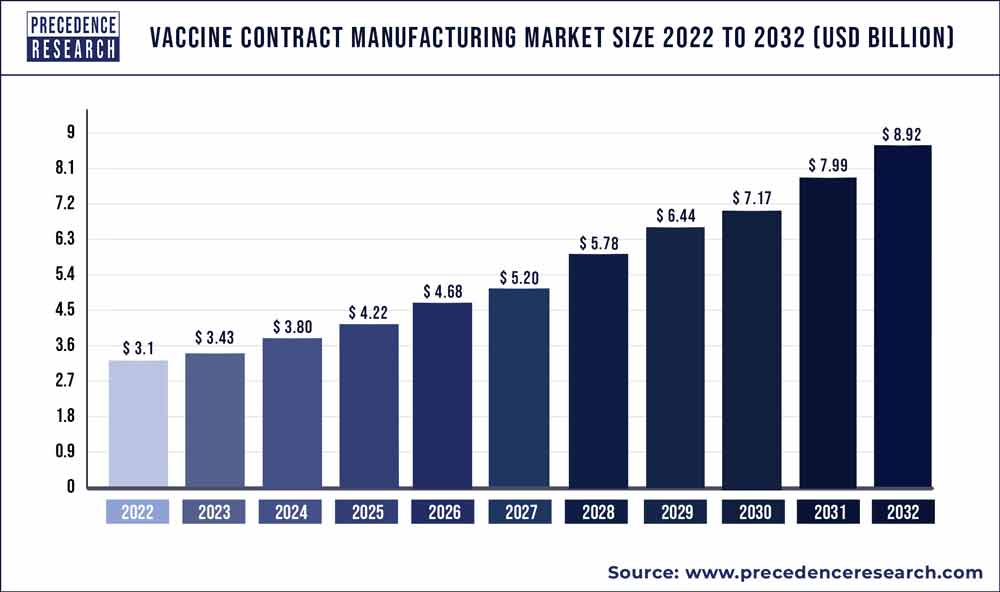
According to a recent research report titled ” Vaccine Contract Manufacturing Market (By Vaccine Type: Attenuated, Inactivated, Subunit-based, Toxoid-based, DNA-based; By Workflow: Downstream, Upstream; By Application: Human Use, Veterinary) – Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2023-2032″ published by Precedence Research, the global vaccine contract manufacturing market size is projected to touch around USD 8.92 billion by 2032 and growing at a CAGR of 11.2% over the forecast period 2023 to 2032. This comprehensive study examines various factors and their impact on the growth of the vaccine contract manufacturing market.
Key Takeaways
- North America led the market with the highest market share of 42% in 2022.
- Asia-Pacific is expected to grow at the fastest CAGR during the forecast period.
- By Vaccine Type, the attenuated vaccines segment has held the largest revenue share of 28% in 2022.
- By Vaccine Type, the inactivated inoculations segment is projected to expand at a remarkable CAGR of 12.7% during the projected period.
- By Workflow, the downstream segment generated more than 68% of revenue share in 2022.
- By Workflow, the upstream segment is anticipated to grow at the fastest CAGR over the projected period.
- By Application, the human-use segment held the major revenue share of 69% in 2022.
- By Application, the veterinary segment is estimated to grow at a noteworthy CAGR of 13.3% over the predicted period.
Vaccine Contract Manufacturing Market Overview:
The Vaccine Contract Manufacturing Market is a dynamic sector within the pharmaceutical industry that involves outsourcing the production of vaccines to specialized manufacturers. This strategic move allows pharmaceutical companies to focus on research, development, and marketing while leveraging the expertise and capabilities of contract manufacturing organizations (CMOs) for the large-scale production of vaccines. The market plays a crucial role in meeting the growing global demand for vaccines, especially in times of pandemics and emerging infectious diseases.
Furthermore, the research report provides specific segmentations based on regions (countries), companies, and all market segments. This analysis offers insights into the growth and revenue trends during the historical period of 2017 to 2032, as well as the projected period. By understanding these segments, it becomes possible to identify the significance of different factors that contribute to market growth.
Download a Free Copy of Our Latest Sample Report@ https://www.precedenceresearch.com/sample/3421
Growth Factors:
Several factors contribute to the growth of the Vaccine Contract Manufacturing Market. Firstly, the increasing prevalence of infectious diseases and the ongoing efforts to combat pandemics have led to a rising demand for vaccines globally. Additionally, advancements in biotechnology and vaccine development technologies have spurred innovation in manufacturing processes, making contract manufacturing an attractive option for pharmaceutical companies. Furthermore, the need for cost-effective and scalable vaccine production, coupled with regulatory support for outsourcing, has fueled the expansion of this market.
Vaccine Contract Manufacturing Market Scope
| Report Coverage | Details |
| Growth Rate from 2023 to 2032 | CAGR of 11.2% |
| Market Size in 2023 | USD 3.43 Billion |
| Market Size by 2032 | USD 8.92 Billion |
| Largest Market | North America |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Vaccine Type, By Workflow, and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Also read: Tuberculosis Diagnostics Market Size To Attain USD 3.67 Billion By 2032
Major Key Points Covered in Report:
Executive Summary: It includes key trends of the electric vehicle fuel cell market related to products, applications, and other crucial factors. It also provides analysis of the competitive landscape and CAGR and market size of the electric vehicle fuel cell market based on production and revenue.
Production and Consumption by Region: It covers all regional markets to which the research study relates. Prices and key players in addition to production and consumption in each regional market are discussed.
Key Players: Here, the report throws light on financial ratios, pricing structure, production cost, gross profit, sales volume, revenue, and gross margin of leading and prominent companies competing in the Electric vehicle fuel cell market.
Market Segments: This part of the report discusses product, application and other segments of the electric vehicle fuel cell market based on market share, CAGR, market size, and various other factors.
Research Methodology: This section discusses the research methodology and approach used to prepare the report. It covers data triangulation, market breakdown, market size estimation, and research design and/or programs.
Vaccine Contract Manufacturing Market Opportunities:
The Vaccine Contract Manufacturing Market presents numerous opportunities for both CMOs and pharmaceutical companies. Collaborative partnerships between manufacturers and developers can expedite the vaccine production timeline, ensuring timely responses to public health crises. The market also offers opportunities for CMOs to invest in cutting-edge manufacturing technologies, such as single-use systems and continuous manufacturing, enhancing efficiency and flexibility. Moreover, the increasing emphasis on personalized vaccines and the development of mRNA-based vaccines open new avenues for contract manufacturing services.
Vaccine Contract Manufacturing Market Challenges:
Despite its promising outlook, the Vaccine Contract Manufacturing Market faces certain challenges. Quality control and regulatory compliance are paramount in vaccine production, and ensuring consistency across different manufacturing facilities can be challenging. Supply chain disruptions, as witnessed during the COVID-19 pandemic, underscore the importance of robust contingency plans. Additionally, the complex nature of some vaccines, such as those requiring live attenuated viruses, poses technical challenges for large-scale production. Balancing cost-effectiveness with maintaining high-quality standards remains an ongoing challenge for both CMOs and pharmaceutical companies operating in this market.
Market Key Players
The report incorporates company profiles of key players in the market. These profiles encompass vital information such as product portfolio, key strategies, and a comprehensive SWOT analysis for each player. Additionally, the report presents a matrix illustrating the presence of each prominent player, enabling readers to gain actionable insights. This facilitates a thoughtful assessment of the market status and aids in predicting the level of competition in the vaccine contract manufacturing market.
Some of the prominent players in the vaccine contract manufacturing market include
- Lonza
- FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
- Ajinomoto Althea, Inc.
- Merck KgaA
- Cytovance Biologics
- Catalent, Inc.
- IDT Biologika GmbH
- Albany Molecular Research, Inc.
- PRA Health Sciences
- ICON plc.
- Pharmaceutical Product Development, LLC
- Cobra Bio
- Paragon Bioservices, Inc.
Market Segmentations
By Vaccine Type
- Attenuated
- Inactivated
- Subunit-based
- Toxoid-based
- DNA-based
By Workflow
- Downstream
- Upstream
By Application
- Human Use
- Veterinary
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Table of Content:
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Vaccine Contract Manufacturing Market
5.1. COVID-19 Landscape: Vaccine Contract Manufacturing Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Vaccine Contract Manufacturing Market, By Vaccine Type
8.1. Vaccine Contract Manufacturing Market, by Vaccine Type, 2023-2032
8.1.1 Attenuated
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Inactivated
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Subunit-based
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Toxoid-based
8.1.4.1. Market Revenue and Forecast (2020-2032)
8.1.5. DNA-based
8.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Vaccine Contract Manufacturing Market, By Workflow
9.1. Vaccine Contract Manufacturing Market, by Workflow, 2023-2032
9.1.1. Downstream
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Upstream
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Electrolyte
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Separator
9.1.4.1. Market Revenue and Forecast (2020-2032)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Vaccine Contract Manufacturing Market, By Application
10.1. Vaccine Contract Manufacturing Market, by Application, 2023-2032
10.1.1. Human Use
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Veterinary
10.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global Vaccine Contract Manufacturing Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.1.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.1.3. Market Revenue and Forecast, by Application (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.1.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.1.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.1.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.1.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.6.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.6.3. Market Revenue and Forecast, by Application (2020-2032)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.2.7.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.2.7.3. Market Revenue and Forecast, by Application (2020-2032)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.6.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.6.3. Market Revenue and Forecast, by Application (2020-2032)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.3.7.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.3.7.3. Market Revenue and Forecast, by Application (2020-2032)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.6.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.6.3. Market Revenue and Forecast, by Application (2020-2032)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.4.7.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.4.7.3. Market Revenue and Forecast, by Application (2020-2032)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.5.3. Market Revenue and Forecast, by Application (2020-2032)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.5.4.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.5.4.3. Market Revenue and Forecast, by Application (2020-2032)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Vaccine Type (2020-2032)
11.5.5.2. Market Revenue and Forecast, by Workflow (2020-2032)
11.5.5.3. Market Revenue and Forecast, by Application (2020-2032)
Chapter 12. Company Profiles
12.1. Lonza
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Ajinomoto Althea, Inc.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Merck KgaA
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Cytovance Biologics
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Catalent, Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. IDT Biologika GmbH
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Albany Molecular Research, Inc.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. PRA Health Sciences
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. ICON plc.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Why should you invest in this report?
This report presents a compelling investment opportunity for those interested in the global vaccine contract manufacturing market. It serves as an extensive and informative guide, offering clear insights into this niche market. By delving into the report, you will gain a comprehensive understanding of the various major application areas for vaccine contract manufacturing. Furthermore, it provides crucial information about the key regions worldwide that are expected to experience substantial growth within the forecast period of 2023-2030. Armed with this knowledge, you can strategically plan your market entry approaches.
Moreover, this report offers a deep analysis of the competitive landscape, equipping you with valuable insights into the level of competition prevalent in this highly competitive market. If you are already an established player, it will enable you to assess the strategies employed by your competitors, allowing you to stay ahead as market leaders. For newcomers entering this market, the extensive data provided in this report is invaluable, providing a solid foundation for informed decision-making.
Some of the key questions answered in this report:
- What is the size of the overall Vaccine contract manufacturing market and its segments?
- What are the key segments and sub-segments in the market?
- What are the key drivers, restraints, opportunities and challenges of the Vaccine contract manufacturing market and how they are expected to impact the market?
- What are the attractive investment opportunities within the Vaccine contract manufacturing market?
- What is the Vaccine contract manufacturing market size at the regional and country-level?
- Who are the key market players and their key competitors?
- What are the strategies for growth adopted by the key players in Vaccine contract manufacturing market?
- What are the recent trends in Vaccine contract manufacturing market? (M&A, partnerships, new product developments, expansions)?
- What are the challenges to the Vaccine contract manufacturing market growth?
- What are the key market trends impacting the growth of Vaccine contract manufacturing market?
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.pharma-geek.com